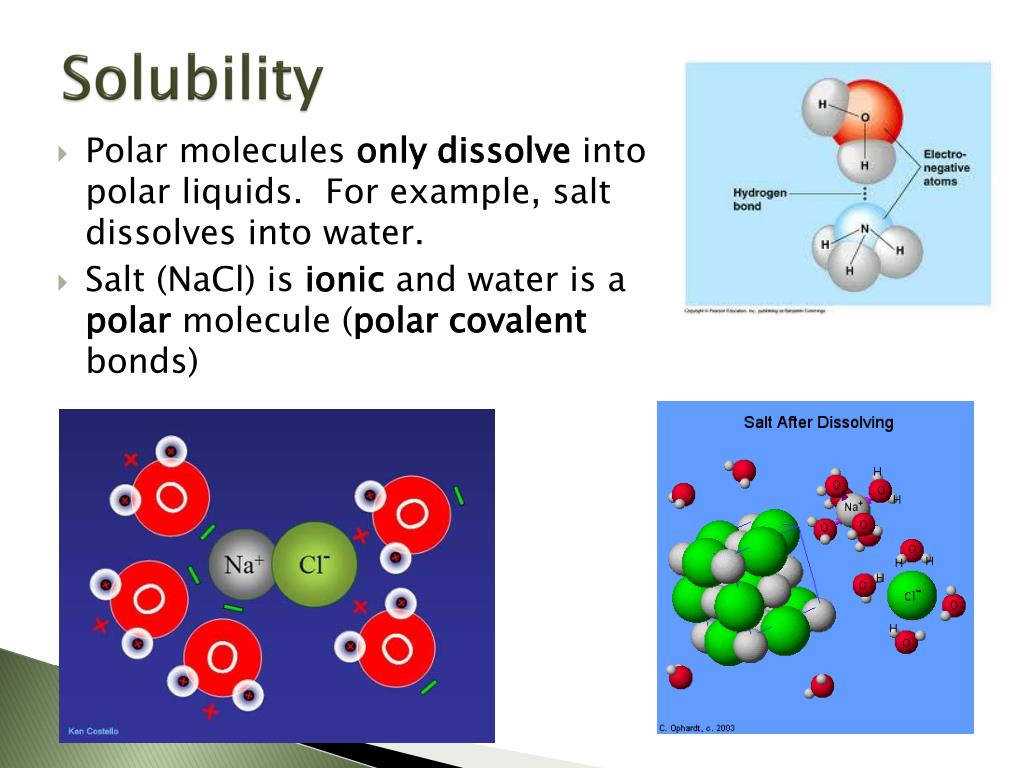
Why Are Ionic Compounds Easily Soluble In Water . predict solubilities of ionic compounds in water using the solubility rules. a overview of solubility as a key property of ionic compounds,. But, except that, here is a simple answer: The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic.
from www.slideserve.com
a overview of solubility as a key property of ionic compounds,. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. But, except that, here is a simple answer: solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. The extent to which a substance. predict solubilities of ionic compounds in water using the solubility rules. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because.
PPT Solubility PowerPoint Presentation, free download ID2493612
Why Are Ionic Compounds Easily Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance. But, except that, here is a simple answer: You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a overview of solubility as a key property of ionic compounds,. predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Compounds AII sodium (Na Why Are Ionic Compounds Easily Soluble In Water predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance. But, except that, here is a simple answer: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. a overview of solubility as a key property of ionic compounds,. You can look up here something. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Why Are Ionic Compounds Easily Soluble In Water You can look up here something about ionic bonding. a overview of solubility as a key property of ionic compounds,. But, except that, here is a simple answer: predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates. Why Are Ionic Compounds Easily Soluble In Water.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Easily Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. solubility is a result of an interaction between. Why Are Ionic Compounds Easily Soluble In Water.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility chart given in the lab Why Are Ionic Compounds Easily Soluble In Water solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. You can look up. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation ID3205890 Why Are Ionic Compounds Easily Soluble In Water predict solubilities of ionic compounds in water using the solubility rules. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. a overview. Why Are Ionic Compounds Easily Soluble In Water.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Why Are Ionic Compounds Easily Soluble In Water a overview of solubility as a key property of ionic compounds,. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideshare.net
Solubility rules and ionic equations 2 Why Are Ionic Compounds Easily Soluble In Water The extent to which a substance. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water,. Why Are Ionic Compounds Easily Soluble In Water.
From socratic.org
What is the chemical equation for HCl dissolving into water and ionizing? Socratic Why Are Ionic Compounds Easily Soluble In Water solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a overview of solubility as a key property of ionic compounds,. But,. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 Why Are Ionic Compounds Easily Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Compounds Easily Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. But, except that, here. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID7002512 Why Are Ionic Compounds Easily Soluble In Water a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. But, except that, here is a simple answer: The extent to which a substance. solubility is a result of an interaction between polar water molecules and the ions that make up. Why Are Ionic Compounds Easily Soluble In Water.
From www.formsbank.com
Solubility Guidelines For Common Ionic Compounds In Water printable pdf download Why Are Ionic Compounds Easily Soluble In Water The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. But, except that, here is a simple answer: ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID2871797 Why Are Ionic Compounds Easily Soluble In Water The extent to which a substance. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. But, except. Why Are Ionic Compounds Easily Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Compounds Easily Soluble In Water a overview of solubility as a key property of ionic compounds,. But, except that, here is a simple answer: ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid separate. Why Are Ionic Compounds Easily Soluble In Water.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure & Properties翰林国际教育 Why Are Ionic Compounds Easily Soluble In Water You can look up here something about ionic bonding. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. But, except that, here. Why Are Ionic Compounds Easily Soluble In Water.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Foundation Why Are Ionic Compounds Easily Soluble In Water a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. You can look up here something about ionic bonding. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. The extent to. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Stoichiometry PowerPoint Why Are Ionic Compounds Easily Soluble In Water a overview of solubility as a key property of ionic compounds,. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. The. Why Are Ionic Compounds Easily Soluble In Water.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download ID3076492 Why Are Ionic Compounds Easily Soluble In Water solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. You can look up here something about ionic bonding. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. a overview of solubility as a key property of ionic compounds,. But, except that,. Why Are Ionic Compounds Easily Soluble In Water.